Abstract
Introduction:
P2Y12 receptor (P2Y12R) defect is a rare hemorrhagic disorder characterized by mild to moderate bleeding diathesis with easy bruising, mucosal bleedings, and excessive post-operative hemorrhage. It is due to mutations of the P2YR12 gene resulting in impairment of platelet responses to adenosine 5'-diphosphate (ADP) and subsequently to any other agonist as ADP is secreted from the dense granules during platelet activation. The P2Y12R plays a key role in platelet aggregation by amplifying the platelet activation process which is necessary to ensure appropriate primary hemostasis. The activation of the P2Y12R by ADP results in inhibition of adenylyl cyclase (AC) and in activation of phosphoinositide 3-kinase (PIK3), which amplifies and sustains the platelet aggregation response. To date only 12 pathogenic variants in the P2YR12 gene are listed in the Human Gene Mutation Database (HGMD®) (10 missense/nonsense mutations and 2 small deletions). Seven missense mutations are described in the literature in patients suffering from bleeding disorders of various severity. Autosomal recessive (homozygous and compound heterozygous pathogenic variants) as well as autosomal dominant (only one heterozygous pathogenic variant) inheritance has been reported.
Patients and Methods:
We investigated an 8 year old index patient who came to our outpatient clinic to investigate an increased bleeding tendency. He suffered from easy bruising and reported increased bleeding after tonsillectomy. His twin brother was also affected regarding bleeding disorder. Their father suffered sometimes from epistaxis, their mother and three older siblings were clinically unaffected.
Platelet functions were assessed by light transmission aggregometry using citrated PRP. Membrane glycoprotein quantification, fibrinogen binding, von Willebrand factor (VWF) binding and the VASP assay were performed by flow cytometry. Molecular genetic analysis (Sanger sequencing) was performed for all exons including UTR regions and splice sites of the P2RY12-gene (NM_002788.4) for the twins and their parents.
Results:
The index patient and his brother showed normal values for platelet count, aPTT, INR, FXIII and VWF parameters. Both twins showed impaired platelet aggregation after stimulation with collagen, ADP and epinephrine. For both the index patient and his twin brother ADP (4-20 µM and 4-10 µM respectively) induced aggregation was markedly reduced and rapidly reversible.
Flow cytometry revealed severely decreased fibrinogen binding for the index patient and his twin brother after stimulation with ADP (0.25-5.0 μmol/l) compared to control platelets. For CD42a/b (GP Ib/IX-complex), CD41 (GP IIb/IIIa-complex), ristocetin-induced VWF-binding, CD62- and CD63-expression, normal values were measured. The VASP assay revealed a strong defect with a PRI value of patient's platelets as low as 1.2% and 1% respectively (normal range > 69%).
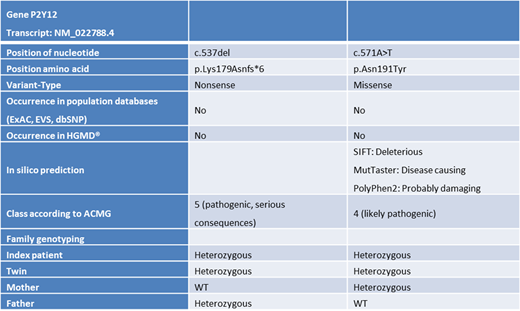
Molecular genetic analysis revealed two novel variants in the P2YR12 coding sequence (Tab.1).
Conclusion:
We have identified two novel variants in the P2YR12 gene with pathogenic predictions in a compound heterozygous state in twin brothers suffering from a chronic bleeding disorder. Flow cytometry analyses (fibrinogen binding) and VASP analyses are valuable tools to diagnose a platelet ADP-receptor defect.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal